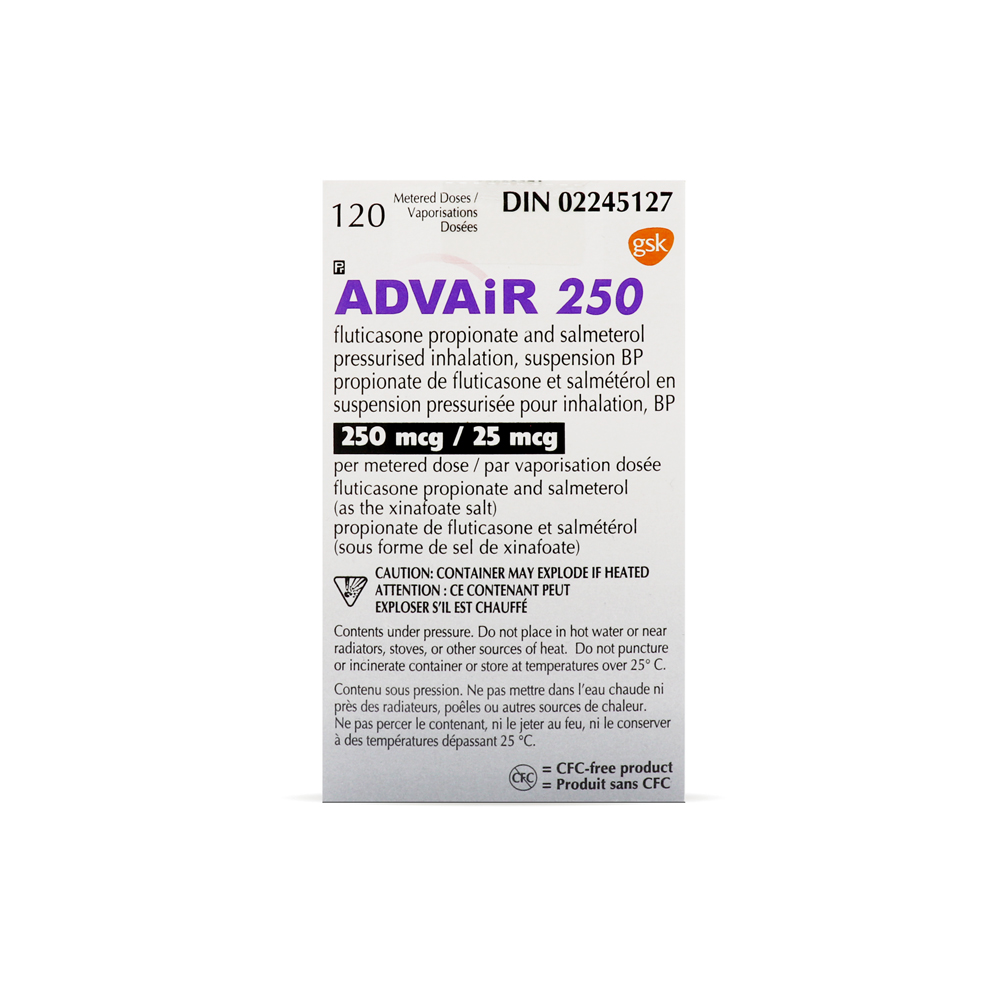
Advair MDI (Fluticasone Salmeterol)
Brand: Advair MDI
Active Ingredient(s): Fluticasone Salmeterol
Pack Size: 120 units – 120 units Metered Dose Inhaler
Store at room temperature.
Manufacturer: GlaxoSmithKline (Canada)
Alternatives: Advair Diskus – PMS Fluticasone Propionate / Salmeterol DPI
*A valid prescription is required for Rx items.
Description
Advair MDI (Fluticasone Salmeterol) is a combination of an inhaled corticosteroid (Fluticasone) and a bronchodilator (Salmeterol). It is typically used to treat asthma (a condition in which your airways narrow and swell and may produce extra mucus), and chronic obstructive pulmonary disease (COPD), including chronic bronchitis (inflammation of the lining that carries air to and from your lungs) and emphysema (damage to the walls of the air sacs (alveoli) of the lungs). COPD is a chronic inflammatory lung disease that causes obstructed airflow from the lungs. It may also have other uses.
Additional information
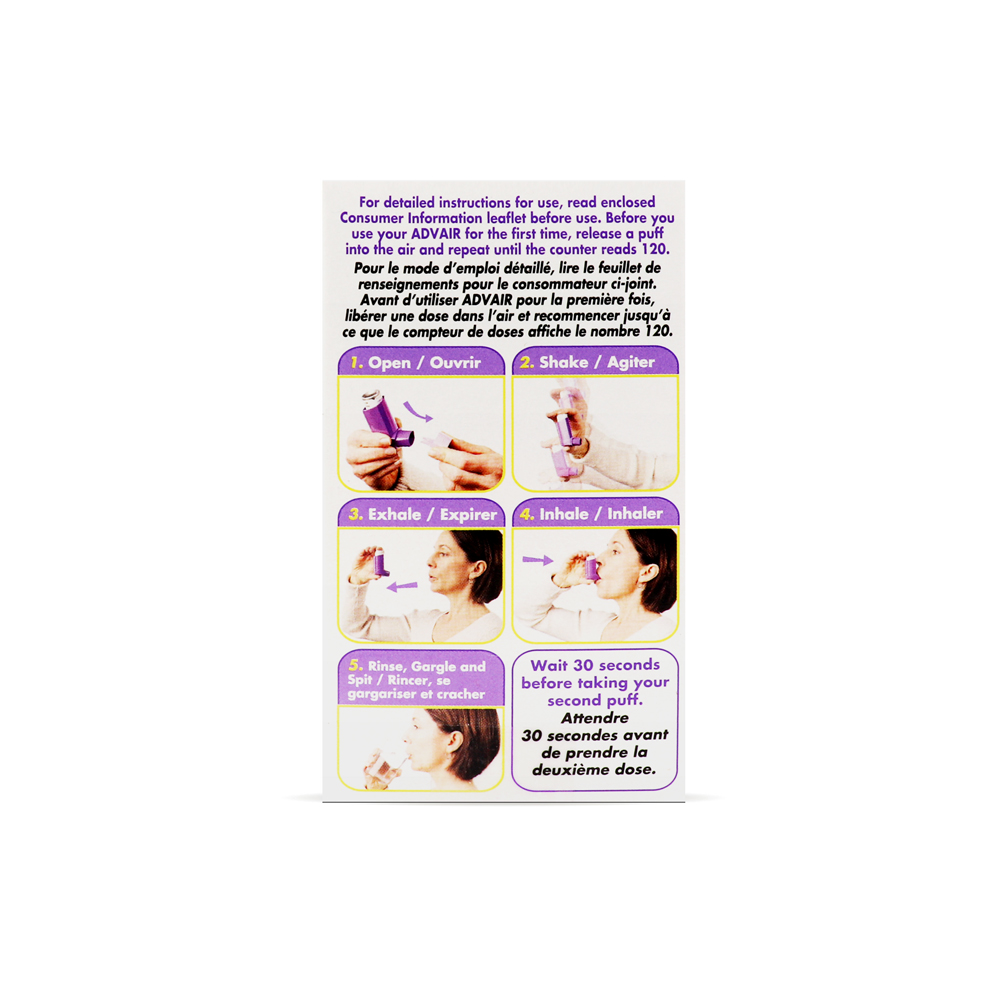
Advair MDI (Fluticasone Salmeterol) is typically inhaled every 12 hours. However, your doctor or pharmacist may have suggested a different schedule that is more appropriate for you. Shake well for 5 seconds before each spray. Prime with 4 test sprays (into the air and away from face) before using for the first time. Priming means making the inhaler ready to use. Important: Follow the instructions on the label. Do not use more of this product, or more often, than prescribed. If you forget a dose, contact your pharmacist. WARNING: Use with caution in patients with major risk factors for decreased bone mineral count (e.g., prolonged immobilization, family history of osteoporosis (weakened bones), postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants or oral corticosteroids), long-term use of inhaled corticosteroids have been associated with decreases in bone mineral density. Use with caution in patients with diabetes (may increase blood sugar level), hyperthyroidism (overactive thyroid, a gland in the neck), or seizure disorders. In addition to its desired action, Advair MDI (Fluticasone Salmeterol) may cause some side effects, notably: It may cause respiratory symptoms or infections (e.g., pharyngitis or sore throat), rhinitis (runny nose), or pneumonia (an infection that inflames the air sacs in one or both lungs). It may cause voice hoarseness, throat irritation, or oral thrush (fungal infection of the mouth). It may cause headaches, nausea, trouble sleeping, or dizziness. It may cause a faster heartbeat than usual. Contraindications: Hypersensitivity to Fluticasone, Salmeterol, or any component of the formulation. Do not use in case of status asthmaticus (severe asthma attack), acute episodes of asthma or COPD. Taking Advair MDI (Fluticasone Salmeterol) during pregnancy may be harmful for the baby. Women of childbearing potential should use an effective contraceptive method. Pregnant women should not use this product. If you're planning a pregnancy or become pregnant or breastfeeding, contact your family doctor. A treatment with Advair MDI (Fluticasone Salmeterol) requires regular monitoring by a doctor. Be sure to see your doctor for all regularly scheduled appointments.Advair MDI (Fluticasone Salmeterol)
Warning
Each person may react differently to a treatment. If you think this medication may be causing side effects (including those described here, or others), talk to your doctor or pharmacist. He or she can help you to determine whether or not the medication is the source of the problem.
There are no reviews yet.







Reviews